Retail Price
Wrist braces and assistive eating utensils can range in cost from under $5 to over $160. We expect our device to retail for around $20, in line with other braces on sites such as Amazon.com
Market Size
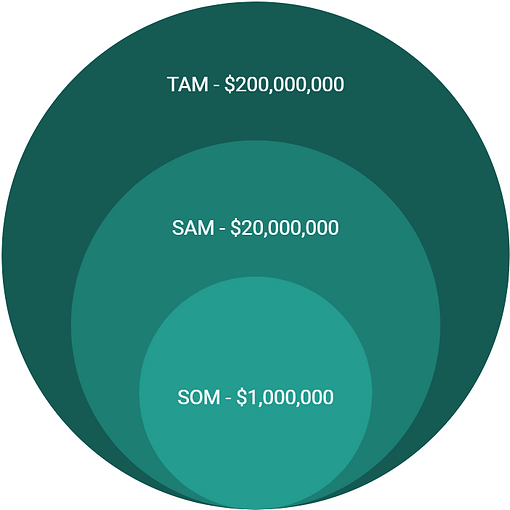
With a retail price of $20, and a total patient population of 10 million, our Total Addressable Market (TAM) will be $200,000,000. Focusing on just the US patient population of 1 million, our Serviceable Available Market (SAM) will be $20,000,000. Lastly, we expect, with the unique helpfulness of our device, that we should be able to sell our product to 5% of the US patient population, or 50,000 people. This will give us a Serviceable Obtainable Market (SOM) of $1,000,000.
Value to Customer
According to the Michael J. Fox Foundation for Parkinson’s Research, the yearly cost of Parkinson’s disease due to non-medical costs is $26.5 billion. This is due to family caregiver time, lost wages, and early retirement. One patient described how they had to pay to cover household tasks that they could no longer physically accomplish.
Our problem thesis aptly summarizes the value that our device will provide to our customers.
Mild to Moderate Parkinson's Patients will use a utensil grip-stability device in order to decrease the rate of dropping and increase ability to write and cook and maintain autonomy.
As shown throughout this report, our device will allow for greater patient autonomy through effective improvement in ability to grip objects. The unique helpfulness of our device in addressing the everyday needs of Parkinson’s patients combined with the low overall cost will allow us to successfully pursue these market goals, and expand into new markets in the future.
Regulatory Pathway
Establishment Registration
The first step to getting our device approved by the FDA will be registering ourselves as an establishment. This is accompanied by an annual fee of $5,236 (as of 2020).
Device Registration
Our device is classified as Class I, due to its low risk to the patient and external use. However, unlike many other Class I devices, ours is not exempt from the 510(k) device review. This is due to the fact that, although it is similar in appearance to a limb brace (an existing exempt device under 890.3475), it does not have the same intended use. Following our registration as an establishment, we will submit our 510(k) along with the $2,899 fee, and await our approval from the FDA.
Following Approval
The approval period for a 510(k) is 177 days on average. Following this process, we will be able to move on to manufacturing.
The Market
Market Research
Assistive Parkinson’s Devices
Parkinson’s disease afflicts more than 10 million people worldwide, and over 1 million people in the US. The market for assistive Parkinson’s devices is vast, covering a variety of devices from walkers and canes, to gyroscopic spoons. The market for our device will be a subset of this, and focus specifically on patients in the US. As our company continues to grow, we will look to expand to overseas markets.
Manufacturing
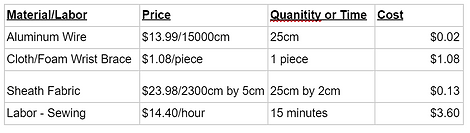
Our manufacturing process is expected to cost less than $5 per unit. A price breakdown is included below.
Total Manufacturing Cost - $4.83 per device